Obesity is typically associated with high rates of inflammatory gut, skin, cardiometabolic disorders (high cholesterol, high triglycerides, diabetes), chronic pain and NAFLD.
Pharma drugs, without ongoing lifestyle support and modification, have been proven ineffective to reverse or prevent “Cobesity” - the inflammatory diseases associated with Obesity.
80% of drugs fail to pass FDA and reach patients due to problems with efficacy and/or safety. And that, to us, shows just how much we don’t know about what targets to pick, what else they might do when we affect them, what other things the drug compounds might do when we use them.
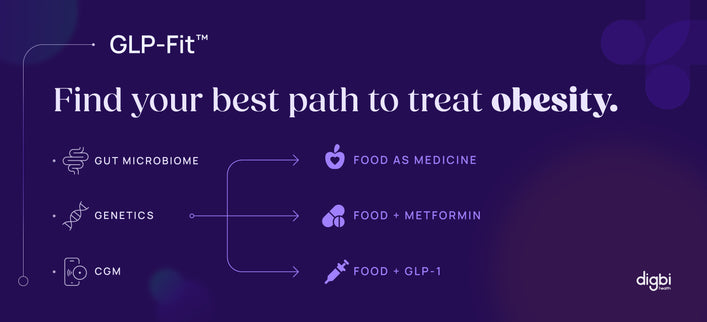
Digbi Health believes we can use genetic, gut microbiome, inexpensive commonly measured blood metabolite, medicine consumed and lifestyle data to find a way to understand and predict these things better and significantly reduce the cost of turning a molecular entity into successful inexpensive therapy.
The gut microbiome has been implicated in most of these inflammatory diseases, even autism, and cancer. Growing evidence from animal and human studies suggests that gut microbiome has causal effects through various mechanisms such as regulating host gene expression or by producing good and inflammatory metabolites (chemicals) that get absorbed back from the gut and circulate in the blood. And because the gut biome is primarily not shaped by host genetics but by modifiable lifestyle factors such as food, this presents an opportunity to intervene.
One of the proven ways to reduce costs is to use genome data to help researchers to determine if the drug targets are relevant to the disease at the start, and drugs with genetic information have shown twice as likely to be approved. We can further optimize drug discovery by intelligently combining molecular data about the microbiome, genome, blood metabolites and proteins, coupled with phenotype and lifestyle measurements to create a ‘360-degree” profile which may allow, in some cases, to skip animal testing and move straight to human trials.
The ability to start drug discovery with this “deep’ profile and information is particularly useful for finding probiotic, prebiotic and synbiotic related therapies.
Digbi Health is continuously developing cohorts that suffer from Cobesity and collecting their genetics, longitudinal lifestyle data (food, exercise, stress, cravings, sleep), common lipids, sugar blood metabolites data related to these diseases and periodically gut microbiome data.
We believe this rich genetic, gut microbiome, molecular and lifestyle profiles will allow drug companies to prioritize human-relevant targets for development, rather than focus on microbial targets with therapeutic value in animal models that many times turn out to be rare in the human microbiome, or that act through different metabolic pathways in people. Interventions to modify bacteria can also start directly in people.
The development of microbiome-related therapeutics faces many challenges, including the need to establish causal mechanisms. However, even if these are unknown, we are able to use human microbiome data to devise food-based (prebiotic) therapies. For example, for patients that have a high cardiovascular risk as demonstrated by their blood LDL levels, and familial hypercholesterolemia gene variation, our precision care software model generates meal plans include/exclude food that targets limiting growth of gut bacteria that synthesize the metabolite trimethylamine N-oxide (TMAO) by reducing foods rich in choline (e.g.brussel sprouts). It has been suggested that high plasma levels of TMAO increases risk of arterial plaque stability leading to increased risk of cardiovascular disease; reducing TMAO production could, therefore, help to lower disease risk. We track changes to the microbiome, blood metabolites to ensure our food-based targeting is being effectuated correctly.
Having time series, multiple types of data on the same person is proven effective in defining more exact targets and for discovering new disease biomarkers and drug targets. “360 Degree” profiling also allows the disease state or treatment response to be modeled on a continuum vs. setting arbitrary thresholds that categorize people as responders or non-responders, for example. This might lead to better estimates of disease risk and better prioritization of people to treatments and randomized controlled trials. The microbiome can also affect the efficacy of pharmaceutical drugs. Bacterial enzymes, for example, can metabolize the Parkinson’s disease drug L-DOPA, and gut bacteria can affect a person’s response to cancer immunotherapy. Dietary changes targeting bacteria that interfere with drug metabolism could, therefore, be effective supplements to existing treatments. The regulatory path for approving such microbiome-nutrition interventions is much easier than for conventional pharmaceutical products.
The approach is to collect dietary, medicine consumption, genetics and microbiome data from many individuals, develop personalized prebiotic (food-based) models of how diet affects the composition of the microbiome and disease state. This data can be used by drug developers to a) create personalized drug dosage recommendations,b) model risk of drug interactions and c) develop successfully personalized medical-grade probiotics and synbiotics.
We can provide multi-ethnic cohorts of thousands today and tens of thousands in a couple of years. This will end the era of capital intensive, risky, arduous, and time-consuming drug discovery.